Page 130 - Teacher Preview Copy
P. 130
.
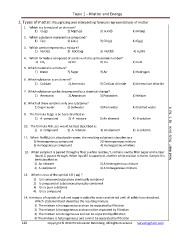
Topic 1 – Matter and Energy
2. Types of matter: Recognizing and interpreting formula representations of matter
1. Which is a formula of an element?
1) Cl2(g) 2) MgCl2(s) 3) H2O(l) 4) HF(aq)
2. Which substance represents a compound?
1) C(s) 2) Co(s) 3) CO (g) 4) O2(g)
3. Which symbol represents a mixture?
1) NaCl(s) 2) NaCl(aq) 3) NaCl(l) 4) H2O(l)
4. Which formula is composed of atoms with the same atomic number?
1) CO2 2) CO 3) Cu 4) CuO
5. Which material is a mixture?
1) Water 2) Sugar 3) Air 4) Hydrogen
6. Which substance is an element?
1) Calcium 2) Ammonia 3) Calcium chloride 4) Ammonium chloride
7. Which substance can be decomposed by a chemical change?
1) Ammonia 2) Aluminum 3) Potassium 4) Helium
8. Which of these contains only one substance? 3) Rain water 4) Distilled water
2) Saltwater
1) Sugar water
9. The formula N2(g) is be best classified as
1) A compound 2) A mixture 3) An element 4) A solution
10. The formula AlBr3 (s) would be best described as
1) A compound 2) A mixture 3) An element 4) A solution lp? Study Book Pg 5 Set 8
11. When NaNO3(s) is dissolved in water, the resulting solution is classifies as a
1) Heterogeneous compound 3) Heterogeneous mixture
2) Homogeneous compound 4) Homogeneous mixture
12. When sample X is passed through a filter a white residue, Y, remains on the filter paper and a clear
liquid, Z, passes through. When liquid Z is vaporized, another white residue remains. Sample X is
best classified as
1) An element 3) A heterogeneous mixture Need He
2) A compound 4) A homogeneous mixture
13. Which is true of the symbol KCl ( aq) ?
1) It is composed substances chemically combined
2) It composed of substances physically combined
3) It is a pure substance
4) It is a compound
14. A mixture of crystals of salt and sugar is added to water and stirred until all solids have dissolved.
Which statement best describes the resulting mixture.
1) The mixture is homogeneous and can be separated by filtration
2) The mixture is homogeneous and cannot be separated by filtration
3) The mixture is heterogeneous and can be separated by filtration
4) The mixture is heterogeneous and cannot be separated by filtration
120 Copyright © 2010 E3 Scholastic Publishing. All Rights Reserved. Survivingchem.com
Topic 1 – Matter and Energy
2. Types of matter: Recognizing and interpreting formula representations of matter
1. Which is a formula of an element?
1) Cl2(g) 2) MgCl2(s) 3) H2O(l) 4) HF(aq)
2. Which substance represents a compound?
1) C(s) 2) Co(s) 3) CO (g) 4) O2(g)
3. Which symbol represents a mixture?
1) NaCl(s) 2) NaCl(aq) 3) NaCl(l) 4) H2O(l)
4. Which formula is composed of atoms with the same atomic number?
1) CO2 2) CO 3) Cu 4) CuO
5. Which material is a mixture?
1) Water 2) Sugar 3) Air 4) Hydrogen
6. Which substance is an element?
1) Calcium 2) Ammonia 3) Calcium chloride 4) Ammonium chloride
7. Which substance can be decomposed by a chemical change?
1) Ammonia 2) Aluminum 3) Potassium 4) Helium
8. Which of these contains only one substance? 3) Rain water 4) Distilled water
2) Saltwater
1) Sugar water
9. The formula N2(g) is be best classified as
1) A compound 2) A mixture 3) An element 4) A solution
10. The formula AlBr3 (s) would be best described as
1) A compound 2) A mixture 3) An element 4) A solution lp? Study Book Pg 5 Set 8
11. When NaNO3(s) is dissolved in water, the resulting solution is classifies as a
1) Heterogeneous compound 3) Heterogeneous mixture
2) Homogeneous compound 4) Homogeneous mixture
12. When sample X is passed through a filter a white residue, Y, remains on the filter paper and a clear
liquid, Z, passes through. When liquid Z is vaporized, another white residue remains. Sample X is
best classified as
1) An element 3) A heterogeneous mixture Need He
2) A compound 4) A homogeneous mixture
13. Which is true of the symbol KCl ( aq) ?
1) It is composed substances chemically combined
2) It composed of substances physically combined
3) It is a pure substance
4) It is a compound
14. A mixture of crystals of salt and sugar is added to water and stirred until all solids have dissolved.
Which statement best describes the resulting mixture.
1) The mixture is homogeneous and can be separated by filtration
2) The mixture is homogeneous and cannot be separated by filtration
3) The mixture is heterogeneous and can be separated by filtration
4) The mixture is heterogeneous and cannot be separated by filtration
120 Copyright © 2010 E3 Scholastic Publishing. All Rights Reserved. Survivingchem.com