Page 207 - Teacher Preview Copy
P. 207
.
Topic 4: Chemical bonding
20 . Molecular structures and shapes: Recognizing bond and molecular polarity by structures
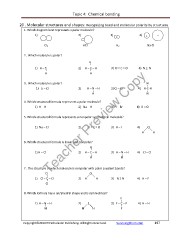
1. Which diagram best represents a polar molecule?
1) 2) 3) 4) + -
Cl2 HCl H2 Na Cl
2. Which molecule is polar?
H
I
1) H – S 2) H – C – H 3) O = C = O 4) N = N
I I
Teacher Preview Copy
H H
H
3. Which molecule is polar? I
1) Li – Cl 2) H – N – H 3) Cl – Cl 4) H-C-H
I I
H H
4. Which structural formula represents a polar molecule?
1) H – H 2) Na – H 3) H – Br 4) O = O
5. Which structural formula represents a nonpolar symmetrical molecule?
Cl Teacher Preview Copy
1) Na – Cl 2) O = C = O 3) H – I 4) O
H H
6. Which structural formula is linear and nonpolar?
H
1) H – Cl I 3) H – N – H 4) Cl – Cl
2) H – C – H
I I
H H
7. The structure of which molecule is nonpolar with polar covalent bonds?
O
I 2) H H 3) N Ξ N 4) H – F
1) Cl – C – Cl
I
Cl
8. Which formula has a tetrahedral shape and is symmetrical?
F
1) H – N – H 2) S I
3) F – C – F
4) H – H
I
I
H H H F
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 197
Topic 4: Chemical bonding
20 . Molecular structures and shapes: Recognizing bond and molecular polarity by structures
1. Which diagram best represents a polar molecule?
1) 2) 3) 4) + -
Cl2 HCl H2 Na Cl
2. Which molecule is polar?
H
I
1) H – S 2) H – C – H 3) O = C = O 4) N = N
I I
Teacher Preview Copy
H H
H
3. Which molecule is polar? I
1) Li – Cl 2) H – N – H 3) Cl – Cl 4) H-C-H
I I
H H
4. Which structural formula represents a polar molecule?
1) H – H 2) Na – H 3) H – Br 4) O = O
5. Which structural formula represents a nonpolar symmetrical molecule?
Cl Teacher Preview Copy
1) Na – Cl 2) O = C = O 3) H – I 4) O
H H
6. Which structural formula is linear and nonpolar?
H
1) H – Cl I 3) H – N – H 4) Cl – Cl
2) H – C – H
I I
H H
7. The structure of which molecule is nonpolar with polar covalent bonds?
O
I 2) H H 3) N Ξ N 4) H – F
1) Cl – C – Cl
I
Cl
8. Which formula has a tetrahedral shape and is symmetrical?
F
1) H – N – H 2) S I
3) F – C – F
4) H – H
I
I
H H H F
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 197