Page 235 - Teacher Preview Copy
P. 235
.
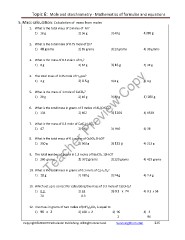
Topic 6: Mole and stoichiometry - Mathematics of formulas and equations
5. Mass calculation: Calculation of mass from moles
1. What is the total mass of 2 moles of Ar?
1) 18 g 2) 36 g 3) 40 g 4) 80 g
2. What is the total mass of 0.75 mole of Zn?
1) 48 grams 2) 85 grams 3) 23 grams 4) 30 grams
3. What is the mass of 0.5 moles of O2?
1) 8 g 2) 64 g 3) 16 g 4) 32 g
Teacher Preview Copy
4. The total mass of 0.25 mole of H2 gas?
1) 2 g 2) 0.5 g 3) 8 g 4) 4 g
5. What is the mass of 1 mole of CaCO3?
1) 20 g 2) 40 g 3) 80 g 4) 100 g
6. What is the total mass in grams of 3 moles of Al2(CrO4)3?
1) 134 2) 402 3) 1206 4) 1530
Teacher Preview Copy
7. What is the mass of 0.3 mole of Ca(C2H3O2)2 ?
1) 47 2) 190 3) 950 4) 38
8. What is the total mass of 0.5 moles of CuSO4.5H2O?
1) 250 g 2) 500 g 3) 125 g 4) 213 g
9. The total number of grams in 1.3 moles of Na2CO3.10H2O?
1) 286 grams 2) 372 grams 3) 220 grams 4) 429 grams
10. What is the total mass in grams of 0.1 mole of C6H12O6?
1) 18 g 2) 180 g 3) 24 g 4) 2.4 g
11. Which set up is correct for calculating the mass of 0.3 mole of Ca(OH)2?
1) 0.3 2) 74 3) 0.3 x 74 4) 0.3 x 58
74 0.3
12. The mass in grams of two moles of (NH4)2CO3 is equal to
1) 96 x 2 2) 108 x 2 3) 96 4) 2
2 96
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 225
Topic 6: Mole and stoichiometry - Mathematics of formulas and equations
5. Mass calculation: Calculation of mass from moles
1. What is the total mass of 2 moles of Ar?
1) 18 g 2) 36 g 3) 40 g 4) 80 g
2. What is the total mass of 0.75 mole of Zn?
1) 48 grams 2) 85 grams 3) 23 grams 4) 30 grams
3. What is the mass of 0.5 moles of O2?
1) 8 g 2) 64 g 3) 16 g 4) 32 g
Teacher Preview Copy
4. The total mass of 0.25 mole of H2 gas?
1) 2 g 2) 0.5 g 3) 8 g 4) 4 g
5. What is the mass of 1 mole of CaCO3?
1) 20 g 2) 40 g 3) 80 g 4) 100 g
6. What is the total mass in grams of 3 moles of Al2(CrO4)3?
1) 134 2) 402 3) 1206 4) 1530
Teacher Preview Copy
7. What is the mass of 0.3 mole of Ca(C2H3O2)2 ?
1) 47 2) 190 3) 950 4) 38
8. What is the total mass of 0.5 moles of CuSO4.5H2O?
1) 250 g 2) 500 g 3) 125 g 4) 213 g
9. The total number of grams in 1.3 moles of Na2CO3.10H2O?
1) 286 grams 2) 372 grams 3) 220 grams 4) 429 grams
10. What is the total mass in grams of 0.1 mole of C6H12O6?
1) 18 g 2) 180 g 3) 24 g 4) 2.4 g
11. Which set up is correct for calculating the mass of 0.3 mole of Ca(OH)2?
1) 0.3 2) 74 3) 0.3 x 74 4) 0.3 x 58
74 0.3
12. The mass in grams of two moles of (NH4)2CO3 is equal to
1) 96 x 2 2) 108 x 2 3) 96 4) 2
2 96
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 225