Page 437 - Teacher Preview Copy
P. 437
.
Reference Tables
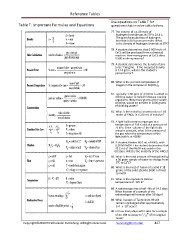
Use equations on Table T for
Table T: Important Formulas and Equations questions that involve calculations.
77. The volume of a 1.00 mole of
hydrogen bromide gas at STP is 22.4 L.
The gram formula mass of hydrogen
bromide is 80.9 gram per mole. What
is the density of hydrogen bromide at STP?
78. A student determines that 0.900 mole of
CaCl2 will be produced from a chemical
reaction. How many grams of CaCl2 does
0.900 mole represents?
79. A student determines the density of zinc
to be 7.56 g/mL. If the accepted density
is 7.14 g/mL, what is the student’s
percent error?
80. What is the percent composition of
oxygen in the compound Ca(ClO3)2.
81. Typically, 0.50 ppm of chlorine is added to
drinking water to help kill disease causing
organisms. How many grams of the solute,
chlorine, would be present in 2000 grams
of drinking water?
82. What is the molarity concentration of .05
moles of KNO3 in a 250 mL of solution?
83. A light bulb contains argon gas at a
temperature of 295 K and at a pressure of
75 kPa. Since volume of the light bulb
remains constant, what is the pressure of
the gas when the temperature of the
light bulb is at 418 K?
84. A student titrates 60.0 mL of HNO3 with
0.30 M NaOH. The student determines that
42.2 mL of the NaOH was used in the
titration. What is the molarity of the HNO3?
85. What is the total amount of heat gained by
a 30 gram sample of water to change from
o
o
5 C to 12 C?
86. What is the heat of fusion of a solid if 18
grams of the solid absorbs 3000 J of heat
to melt?
87. What is the equivalent Celsius
temperature of 125 K?
88. A radioisotope has a half –life of 14.5 days.
What fraction of a sample of the
radioisotope will remain after 58 days?
89. What fraction of Technitium-99 will
remain unchanged after approximately
5
6.4 x 10 years?
90. In how many days will it take for a sample
1
th
of Au-198 to decay to /32 of its original
mass?
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 427
Reference Tables
Use equations on Table T for
Table T: Important Formulas and Equations questions that involve calculations.
77. The volume of a 1.00 mole of
hydrogen bromide gas at STP is 22.4 L.
The gram formula mass of hydrogen
bromide is 80.9 gram per mole. What
is the density of hydrogen bromide at STP?
78. A student determines that 0.900 mole of
CaCl2 will be produced from a chemical
reaction. How many grams of CaCl2 does
0.900 mole represents?
79. A student determines the density of zinc
to be 7.56 g/mL. If the accepted density
is 7.14 g/mL, what is the student’s
percent error?
80. What is the percent composition of
oxygen in the compound Ca(ClO3)2.
81. Typically, 0.50 ppm of chlorine is added to
drinking water to help kill disease causing
organisms. How many grams of the solute,
chlorine, would be present in 2000 grams
of drinking water?
82. What is the molarity concentration of .05
moles of KNO3 in a 250 mL of solution?
83. A light bulb contains argon gas at a
temperature of 295 K and at a pressure of
75 kPa. Since volume of the light bulb
remains constant, what is the pressure of
the gas when the temperature of the
light bulb is at 418 K?
84. A student titrates 60.0 mL of HNO3 with
0.30 M NaOH. The student determines that
42.2 mL of the NaOH was used in the
titration. What is the molarity of the HNO3?
85. What is the total amount of heat gained by
a 30 gram sample of water to change from
o
o
5 C to 12 C?
86. What is the heat of fusion of a solid if 18
grams of the solid absorbs 3000 J of heat
to melt?
87. What is the equivalent Celsius
temperature of 125 K?
88. A radioisotope has a half –life of 14.5 days.
What fraction of a sample of the
radioisotope will remain after 58 days?
89. What fraction of Technitium-99 will
remain unchanged after approximately
5
6.4 x 10 years?
90. In how many days will it take for a sample
1
th
of Au-198 to decay to /32 of its original
mass?
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 427