Page 10 - Surviving Chemistry BFF Homework Helper and Test Prep Preview
P. 10
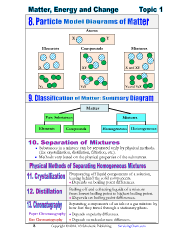
Atoms
X Y
Elements Compounds Mixtures
X XY X and XY
Y 2 Y 2X Y 2 and Y 2X
Y2 X2Y
Matter
Pure Substances Mixtures
Elements Compounds Homogeneous Heterogeneous
Substances in a mixture can be separated only by physical methods.
(Ex: crystallization, distillation, filtration, etc.).
Methods vary based on the physical properties of the substances.
Evaporating off liquid components of a solution,
leaving behind the solid components.
Depends on boiling point differences.
Boiling off and collecting liquids of a mixture
from lowest boiling point to highest boiling point.
Depends on boiling point differences.
Separating components of an ink or a gas mixture by
how fast they travel through a stationary phase.
Paper Chromatography Depends on polarity differences.
Gas Chromatography Depends on molecular mass differences.
8 Copyright © 2014. E3 Scholastic Publishing. SurvivingChem.com