Page 118 - Teacher Preview Copy
P. 118
Worksheet 51 . Topic 11
Set B: Voltaic cell diagrams Objective: To test your ability to determine information
from voltaic cell diagrams
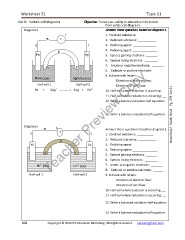
Diagram 4 Answer these questions based on diagram 4.
E 1. Oxidized substance: __________
V 2. Reduced substance: ___________
D F 3. Oxidizing agent: ______________
B 4. Reducing agent: _______________
Pb Ag 5. Species gaining electrons: _______
6. Species losing electrons: ________
7. Anode or negative electrode: _______
A
C 8. Cathode or positive electrode: ______
Teacher Preview Copy
9. Indicate with letters:
PbNO3(aq) Ag(NO3)2(aq) Direction of electron flow:
Half-cell 1 Half-cell 2 Direction of ions flow:
Pb + 2Ag --------- > 2Ag + Pb
+
2+
10. Half-cell where oxidation is occurring: __
11. Half-cell where reduction is occurring: __
12. Write a balance oxidation-half equation:
13. Write a balance reduction-half equation:
Teacher Preview Copy
Diagram 5
b
a V c Answer these questions based on diagram 5.
1. Oxidized substance: __________
Need Help? Study Book Pg 279 Set 31
e 2. Reduced substance: ___________
Ni Co 3. Oxidizing agent: ______________
4. Reducing agent: _______________
d f
5. Species gaining electrons: _______
6. Species losing electrons: ________
2+
2+
Ni (aq) Co (aq) 7. Anode or negative electrode: _______
8. Cathode or positive electrode: ______
Half-cell 1 Half-cell 2 9. Indicate with letters:
Direction of electron flow:
Direction of ions flow:
10. Half-cell where oxidation is occurring: __
11. Half-cell where reduction is occurring: __
12. Write a balanced oxidation-half equation:
13. Write a balance reduction-half equation:
108 Copyright © 2010 E3 Scholastic Publishing. All Rights Reserved. Survivingchem.com
Set B: Voltaic cell diagrams Objective: To test your ability to determine information
from voltaic cell diagrams
Diagram 4 Answer these questions based on diagram 4.
E 1. Oxidized substance: __________
V 2. Reduced substance: ___________
D F 3. Oxidizing agent: ______________
B 4. Reducing agent: _______________
Pb Ag 5. Species gaining electrons: _______
6. Species losing electrons: ________
7. Anode or negative electrode: _______
A
C 8. Cathode or positive electrode: ______
Teacher Preview Copy
9. Indicate with letters:
PbNO3(aq) Ag(NO3)2(aq) Direction of electron flow:
Half-cell 1 Half-cell 2 Direction of ions flow:
Pb + 2Ag --------- > 2Ag + Pb
+
2+
10. Half-cell where oxidation is occurring: __
11. Half-cell where reduction is occurring: __
12. Write a balance oxidation-half equation:
13. Write a balance reduction-half equation:
Teacher Preview Copy
Diagram 5
b
a V c Answer these questions based on diagram 5.
1. Oxidized substance: __________
Need Help? Study Book Pg 279 Set 31
e 2. Reduced substance: ___________
Ni Co 3. Oxidizing agent: ______________
4. Reducing agent: _______________
d f
5. Species gaining electrons: _______
6. Species losing electrons: ________
2+
2+
Ni (aq) Co (aq) 7. Anode or negative electrode: _______
8. Cathode or positive electrode: ______
Half-cell 1 Half-cell 2 9. Indicate with letters:
Direction of electron flow:
Direction of ions flow:
10. Half-cell where oxidation is occurring: __
11. Half-cell where reduction is occurring: __
12. Write a balanced oxidation-half equation:
13. Write a balance reduction-half equation:
108 Copyright © 2010 E3 Scholastic Publishing. All Rights Reserved. Survivingchem.com