Page 119 - Teacher Preview Copy
P. 119
Worksheet 51 .
Set C: Electrolytic cell diagrams Objective: To test your ability to determine information from
electrolytic cell diagrams
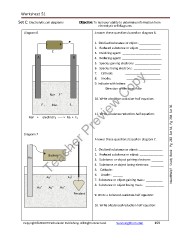
Diagram 6 Answer these questions based on diagram 6.
- +
1. Oxidized substance or object: _____________
C 2. Reduced substance or object _____________
3. Oxidizing agent: _______________________
A B 4. Reducing agent: ________________________
5. Species gaining electrons: ________________
6. Species losing electrons: ________________
Teacher Preview Copy
7. Cathode: _____________________
8. Anode: _____________________
9. Indicate with letters:
Direction of electrons flow:
Na+ F
–
10. Write a balance oxidation-half equation:
F - Na+ F-
11. Write a balance reduction-half equation:
NaF + electricity ----- > Na + F2
+Teacher Preview Copy
Diagram 7
Answer these questions based on diagram 7. Need Help? Study Book Pg 282 Set 34, Pg 284 Set 36
+ - 1. Oxidized substance or object: ______________
Au Battery 2. Reduced substance or object: ______________
3. Substance or object gaining electrons: _______
4. Substance or object losing electrons: ________
5. Cathode: ______
Au Au 6. Anode : ______
+
7. Substance or object gaining mass: __________
Au Au Au 8. Substance or object losing mass: ___________
+
+
+
Pendant 9. Write a balanced oxidation half equation:
10. Write a balanced reduction half equation:
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 109
Set C: Electrolytic cell diagrams Objective: To test your ability to determine information from
electrolytic cell diagrams
Diagram 6 Answer these questions based on diagram 6.
- +
1. Oxidized substance or object: _____________
C 2. Reduced substance or object _____________
3. Oxidizing agent: _______________________
A B 4. Reducing agent: ________________________
5. Species gaining electrons: ________________
6. Species losing electrons: ________________
Teacher Preview Copy
7. Cathode: _____________________
8. Anode: _____________________
9. Indicate with letters:
Direction of electrons flow:
Na+ F
–
10. Write a balance oxidation-half equation:
F - Na+ F-
11. Write a balance reduction-half equation:
NaF + electricity ----- > Na + F2
+Teacher Preview Copy
Diagram 7
Answer these questions based on diagram 7. Need Help? Study Book Pg 282 Set 34, Pg 284 Set 36
+ - 1. Oxidized substance or object: ______________
Au Battery 2. Reduced substance or object: ______________
3. Substance or object gaining electrons: _______
4. Substance or object losing electrons: ________
5. Cathode: ______
Au Au 6. Anode : ______
+
7. Substance or object gaining mass: __________
Au Au Au 8. Substance or object losing mass: ___________
+
+
+
Pendant 9. Write a balanced oxidation half equation:
10. Write a balanced reduction half equation:
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 109