Page 143 - Teacher Preview Copy
P. 143
.
Topic 1 – Matter and Energy
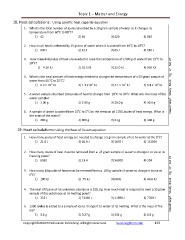
18. Heat calculations: Using specific heat capacity equation
1. What is the total number of joules absorbed by a 10-gram sample of water as it changes its
temperature from 60 C to 80 C?
o
o
1) 42 2) 84 3) 420 4) 840
o
o
2. How much heat is released by 15 grams of water when it is cooled from 40 C to 30 C?
1) 630 J 2) 42 J 3) 63 J 4) 130 J
o
3. How many kilojoules of heat are needed to raise the temperature of a 500 g of water from 15 C to
o
20 C?
1) 4.20 KJ 2) 10.5 KJ 3) 32.0 KJ 4) 105 KJ
4. What is the total amount of heat energy needed to change the temperature of a 65-gram sample of
o
o
water from 40. C to 25 C?
1
1
1) 6.3 x 10 KJ 2) 1.1 x10 KJ 3) 4.1 x 10 KJ 4) 6.8 x 10 KJ Need Help? Study Book Pg 17 Set 27
-2
-1
o o
5. A water sample absorbed 168 joules of heat to change from 10 C to 30 C. What was the mass of the
water sample?
1) 1.00 g 2) 2.00 g 3) 20.0 g 4) 30.0 g
o o
6. A sample of water is cooled from 15 C to 5 C by the removal of 1700 Joules of heat energy. What is
the mass of the water?
1) 200 g 2) 800 g 3) 41 g 4) 400 g
19. Heat calculations: Using the heat of fusion equation
o
1. How many joules of heat energy are needed to change a 5 gram sample of ice to water at the 0 C?
1) 21.0 J 2) 66.8 J 3) 1670 J 4) 11300 J
2. How many Joules of heat must be removed from a 25 gram sample of water to change it to ice at its
freezing point?
1) 8350 2) 13.4 3) 56500 4) 334
3. How many kilojoules of heat must be removed from a 180 g sample of water to change it to ice at
o
0 C?
1) 180 KJ 2) .75 KJ 3) 60 KJ 4) 40.6 KJ
Need Help? Study Book Pg 17 Set 27
4. The heat of fusion of an unknown substance is 220 J/g. How much heat is required to melt a 35 gram
sample of this substance at its melting point?
1) 255 J 2) 73480 J 3) 11690 J 4) 7700 J
5. 1200 Joules is added to a sample of ice to change it to water at its melting. What is the mass of the
ice?
1) 3.6 g 2) 0.27 g 3) 334 g 4) 1.9 g
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 133
Topic 1 – Matter and Energy
18. Heat calculations: Using specific heat capacity equation
1. What is the total number of joules absorbed by a 10-gram sample of water as it changes its
temperature from 60 C to 80 C?
o
o
1) 42 2) 84 3) 420 4) 840
o
o
2. How much heat is released by 15 grams of water when it is cooled from 40 C to 30 C?
1) 630 J 2) 42 J 3) 63 J 4) 130 J
o
3. How many kilojoules of heat are needed to raise the temperature of a 500 g of water from 15 C to
o
20 C?
1) 4.20 KJ 2) 10.5 KJ 3) 32.0 KJ 4) 105 KJ
4. What is the total amount of heat energy needed to change the temperature of a 65-gram sample of
o
o
water from 40. C to 25 C?
1
1
1) 6.3 x 10 KJ 2) 1.1 x10 KJ 3) 4.1 x 10 KJ 4) 6.8 x 10 KJ Need Help? Study Book Pg 17 Set 27
-2
-1
o o
5. A water sample absorbed 168 joules of heat to change from 10 C to 30 C. What was the mass of the
water sample?
1) 1.00 g 2) 2.00 g 3) 20.0 g 4) 30.0 g
o o
6. A sample of water is cooled from 15 C to 5 C by the removal of 1700 Joules of heat energy. What is
the mass of the water?
1) 200 g 2) 800 g 3) 41 g 4) 400 g
19. Heat calculations: Using the heat of fusion equation
o
1. How many joules of heat energy are needed to change a 5 gram sample of ice to water at the 0 C?
1) 21.0 J 2) 66.8 J 3) 1670 J 4) 11300 J
2. How many Joules of heat must be removed from a 25 gram sample of water to change it to ice at its
freezing point?
1) 8350 2) 13.4 3) 56500 4) 334
3. How many kilojoules of heat must be removed from a 180 g sample of water to change it to ice at
o
0 C?
1) 180 KJ 2) .75 KJ 3) 60 KJ 4) 40.6 KJ
Need Help? Study Book Pg 17 Set 27
4. The heat of fusion of an unknown substance is 220 J/g. How much heat is required to melt a 35 gram
sample of this substance at its melting point?
1) 255 J 2) 73480 J 3) 11690 J 4) 7700 J
5. 1200 Joules is added to a sample of ice to change it to water at its melting. What is the mass of the
ice?
1) 3.6 g 2) 0.27 g 3) 334 g 4) 1.9 g
Copyright©2010 E3 Scholastic Publishing. All Rights Reserved. SurvivingChem.com 133