Page 39 - Surviving Chemistry BFF Homework Helper and Test Prep Preview
P. 39
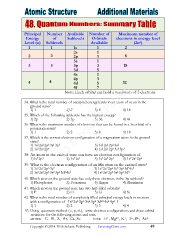
Principal Number Available Number of Maximum number of
Energy of Sublevels Orbitals electrons in energy level
Level (n) Sublevels Available (2n )
2
1 1 1s 1 2
2s 1
2 2 2p 3 8
3s 1
3 3 3p 3 18
3d 5
4s 1
3
4p
4 4 4d 5 32
4f 7
Note: Each orbital can hold a maximum of 2 electrons
34. What is the total number of occupied energy levels in an atom of neon in the
ground state?
1) 1 2) 2 3) 8 4) 18
35. Which of the following sublevels has the highest energy?
1) 2p 2) 3p 3) 3d 4) 4s
(c) E3 Scholastic Publishing
36. What is the maximum number of electrons that can be found in a 3s orbital of a
potassium atom?
1) 1 2) 2 3) 8 4) 18
37. Which is the correct electron configuration of a magnesium atom in the ground
state?
2
6
2
2
6
1
2
1
2
1) 1s 2s 2p 3s 3p 3) 1s 2s 2p 3s
2) 1s 2s 2p 4) 1s 2s 2p 3s 3p 1
2
2
2
2
6
2
6
38. An atom in the excited state can have an electron configuration of
2
1)1s 2p 2) 1s 2s 3) 1s 2s 2p 4) 1s 2s 2p
1
6
2
5
2
2
2
2
2
39. What is the electron configuration of an Mn atom in the excited state?
2
2
6
1) 1s 2s 2p 3s 3) 1s 2s 2p 3s 3p 3d 4s 2
2
2
2
5
2
6
6
2
2
6
2
6
2
6
2) 1s 2s 2p 3s 3p 3d 4s 1 4) 1s 2s 2p 3s 3p 3d 5
2
6
2
6
40. Which atom in the ground state has only three electrons in the 3p sublevel?
1) Phosphorus 2) Potassium 3) Argon 4) Aluminum
41. Which atom in the ground state has two half-filled orbitals?
1) P 2) O 3) Li 4) Si
42. What is the total number of completely filled principal energy levels in an atom
6
10
2
2
1
2
6
2
with a configuration of 1s 2s 2p 3s 3p 3d 4s 4p ?
1) 1 2) 2 3) 3 4) 4
43. Using quantum method ( s, p, d..), write electron configurations and draw orbital
notations for the following atoms and ions.
atoms: C, Al, S, Ar, Ca, Se ions: Li , Mg , K , F , S , As
2-
3-
+
2+
-
+
Copyright © 2014. E3 Scholastic Publishing. SurvivingChem.com 49